Empirical Formula of Butane
Butane is a highly flammable colorless. Butane is a colourless gas that can be easily liquified.
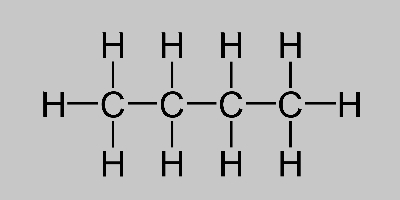
Butane Formula Learn About Butane Isomers And Its Structure
What is the empirical formula of butane.
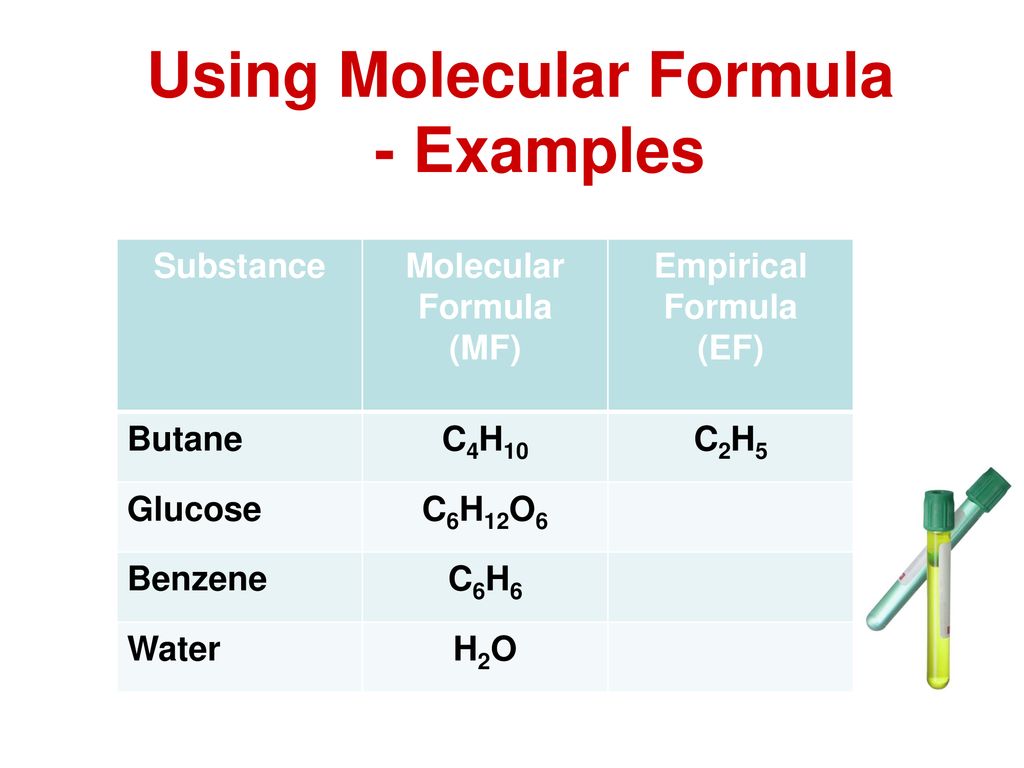
. For every mole of carbon there are two moles of hydrogen. We can simplify the molecular formula C4 H10 which is the formula for butane by dividing the formula What best describes an. The empirical formula tells us the simplest whole-number ratio of the different types of atoms in a compound.
The carbon-to-hydrogen ratio equals 23. Butane or n-butane is an alkane with the formula C₄H₁₀. Precalculus questions and answers.
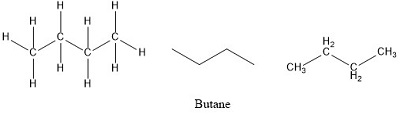
The formula given for butane is a molecular formula. The empirical formula for any molecular is obtained by dividing all the element subscript numbers by the highest. This formula tells us that one molecule of butane contains four carbon atoms and ten hydrogen atoms.
C2H3 is the empirical formula for butane C4H6. The carbon-to-hydrogen ratio equals 23. If you know that the molecular formula of.
This formula does not show. The carbon-to-hydrogen ratio equals 23. The molecular formula of butane is C.
C2H3 is the empirical formula for butane C4H6. A compound has a molar mass of 86 gmol and has a percent composition by mass of 558 C 372 O and 70 H. For every mole of carbon there are two moles of hydrogen.
The molecular formula of butane is C 4 H 10. For example the molecular formula of the compound butane is C4H10. This gives the empirical formula of butane - C 2 H 5.
What is the empirical formula for butane. This is the actual number of atoms of each element in a molecule of butane. What is the empirical formula of butane.
The formula for butane is C 4 H 1 0 because hydrocarbons have the formula C n H 2 n 2 The epirical formula for butane however is C 2 H 5 Was this answer helpful. The empirical formula for C4 H10 is C2 H5. Determine the empirical formula and the molecular formula.
Hint Empirical formula is the simplest formula which provides the lowest whole number ratio of atoms which exist in the compound. FROM THE MOLECULAR FORMULA. The relative number of atoms of every element in the.
Butane is a gas at room temperature and atmospheric pressure. Determine the empirical formula of butane gas if it consists of 8182 carbon and 1818 hy drogendetermine the empirical. What is the empirical formula for butane.
It is an alkane and consists of four aliphatic carbon atoms in a chain. The chemical formula of butane is C4H10.
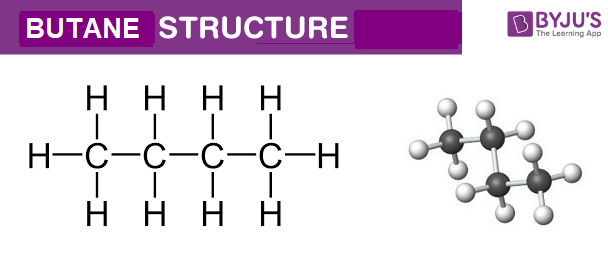
Butane C4h10 Structure Molecular Mass Properties Uses

How To Write The Empirical Formula For Butane C4h10 Youtube

Empirical Formula Of A Compound Ppt Download

Butane Formula Structure What Is Butane Used For Video Lesson Transcript Study Com

Butane Molecular Geometry Hybridization Molecular Weight Molecular Formula Cas Number Bond Pairs Lone Pairs Lewis Structure
0 Response to "Empirical Formula of Butane"
Post a Comment